Investigating Dengue virus structure-function relationship
Dengue infection is a mosquito-borne global healthcare and economic problem. It affects about 400 million people worldwide and is one of the major diseases in Singapore, with Dengue 2 (DENV2) being the most virulent strain among the four serotypes. Currently, there are no effective therapeutics available to tackle Dengue. This highlights the urgent need to understand how Dengue uses conditions prevailing in humans and mosquitoes to its advantage. Evidence suggests that flaviviruses explore an ensemble of morphologies at equilibrium, referred to as viral structural dynamics or “breathing” [1-4] and is an essential aspect of the life cycle of many viruses [1, 5]. These distinct morphologies arise due to large and small scale conformational changes in the organization of surface glycoproteins which results from the virus experiencing perturbations in temperature, pH or host–protein interactions [6](13). DENV surface glycoprotein (E protein), constituting the viral envelope, has four domains (E-DI (residues 1-52; 132-193; 280-296), E-DII (residues 53-131; 194-279), E-DIII (residues 297-394) and the stem domain (residues 395-486)) that connect to a transmembrane region [7, 8].
In our lab, we are investigating dengue viruses’ structure-function relationship at its
different life stages with the hope of providing new clues for developing antiviral strategies.
Using fluorescence and mass spectrometry with molecular dynamics simulations, we have recently
uncovered that the structural dynamics but not the specific virus morphologies are correlated to
Dengue infectivity. These findings reported new vulnerabilities in the Dengue virus as it moves
from the mosquito into an unsuspecting human host and back to mosquito. We demonstrated that
while structures of the Dengue virus change dramatically between the two hosts’
temperatures, neither infectivity nor the structural dynamics of the virus envelope change.
Infectivity and dynamics are only changed once the virus is exposed to fever-like temperatures
of 40 °C. By monitoring how the Dengue virus is influenced by both temperature and the presence
of divalent cations (Ca2+, Mg2+) in the virus environment, the current work has identified the
correlation between viral structural dynamics and the virus infectivity [9,10,11]. Since, these
dynamic events are essential aspects of the Dengue life cycle, this is an important discovery to
counter viral epidemics in Singapore and the world.
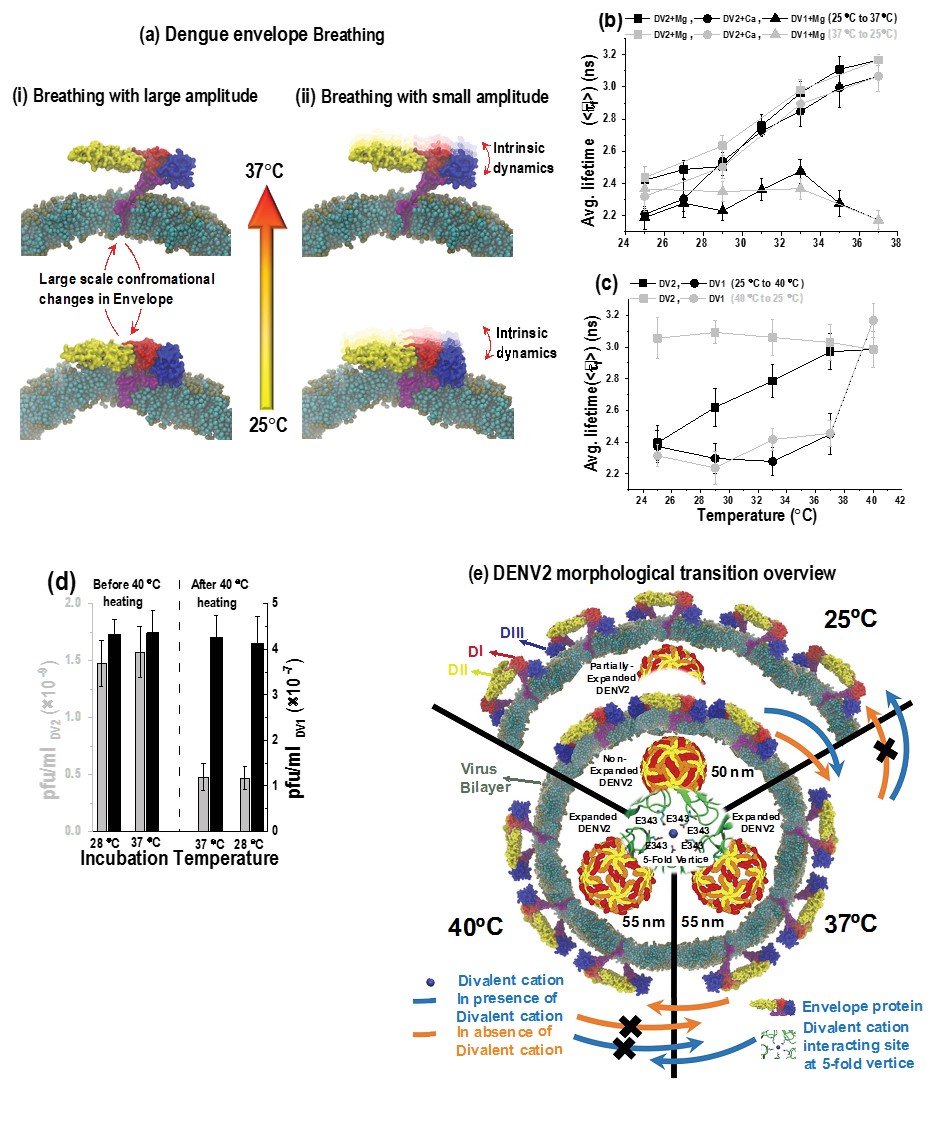
(a) Schematic representation of DENV2 “breathing”. (i) DENV2
undergoes temperature dependent large-scale conformational changes in the
organization of surface E proteins between 25˚C and 37˚C. (ii) DENV2 breathing also encompasses
the E protein structural dynamics that takes place in both smooth and bumpy form at
smaller time and length scales, termed as intrinsic dynamics.
(b) The average fluorescence lifetimes (<τf>) DENV2 in presence of 1 mM
Mg2+-ions (squares) or 2 mM Ca2+-ions (circles) and for DENV1 in presence of 1 mM Mg2+ions
(triangles). The data was collected during temperature increase from 25˚C to 37˚C (black
circles/triangles/squares) followed by decrease from 37˚C to 25˚C (grey
circles/triangles/squares). (c) The average fluorescence lifetimes (<τf>)
for dual-labeled DENV2 (squares) and DENV1 (circles) in the presence of 1 mM Mg2+-ions. The data
was collected during temperature increase from 25˚C to 40˚C (black circles/squares) followed by
decrease from 40˚C to 25˚C (grey circles/squares). (d) Histogram showing Plaque
forming virus titers performed at 1 hour incubation of either DENV2 (grey bars) or DENV1 (black
bars) with BHK21 cells at temperatures of either 28˚C or 37˚C. (e) Figure
showing the morphological and structural changes in Dengue virus at different temperatures. At
25˚C, envelope proteins at the membrane of Dengue virus remain in close proximity. Upon increase
in temperature to ≥37˚C, the distance between envelope proteins and virus increases and lead to
the expansion of Dengue virus. We have uncovered that only in the presence of divalent cations,
Dengue virus undergoes partial shrinkage with decrease in temperature (back to 25˚C).
References
[1] Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. Combined effects of the structural heterogeneity
and dynamics of flaviviruses on antibody recognition. J Virol. 2014;88:11726-37.
[2] Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411:306-15.
[3] Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol. 2008;15:312-7.
[4] Rey FA. Dengue virus: two hosts, two structures. Nature. 2013;497:443-4.
[5] Witz J, Brown F. Structural dynamics, an intrinsic property of viral capsids. Arch Virol. 2001;146:2263-74.
[6] Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13-22.
[7] Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A. 2003;100:6986-91.
[8] Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291-8.
[9] Sharma KK, Lim XX, Tantrimudalige SN, Gupta A, Marzinek JN, Holdbrook D, Lim XYE, Bond JP, Anand GS, Wohland T*. Infectivity of dengue virus serotypes 1 and 2 is correlated to E protein intrinsic dynamics but not to envelope conformations. bioRxiv; DOI https://doi.org/10.1101/3035602018.
[10] Sharma KK, Marzinek JK, Tantirimudalige SN, Bond PJ, Wohland T*. Single-molecule studies of flavivirus envelope dynamics: Experiment and computation. Prog Biophys Mol Biol. 2018 Sep 14. pii: S0079-6107(18)30181-0. doi: 10.1016/j.pbiomolbio.2018.09.001. Review.
[11] Lim XX; Chandramohan A; Lim XYE; Bag N; Sharma KK; Wirawan M; Wohland T; Lok SM; Anand GS*, “Conformational changes in intact dengue virus reveal serotype-specific expansion.”, NATURE COMMUNICATIONS Volume: 8, Article number: 14339 DOI: 10.1038/ncomms14339 Published: 2017.