Confocal FCS
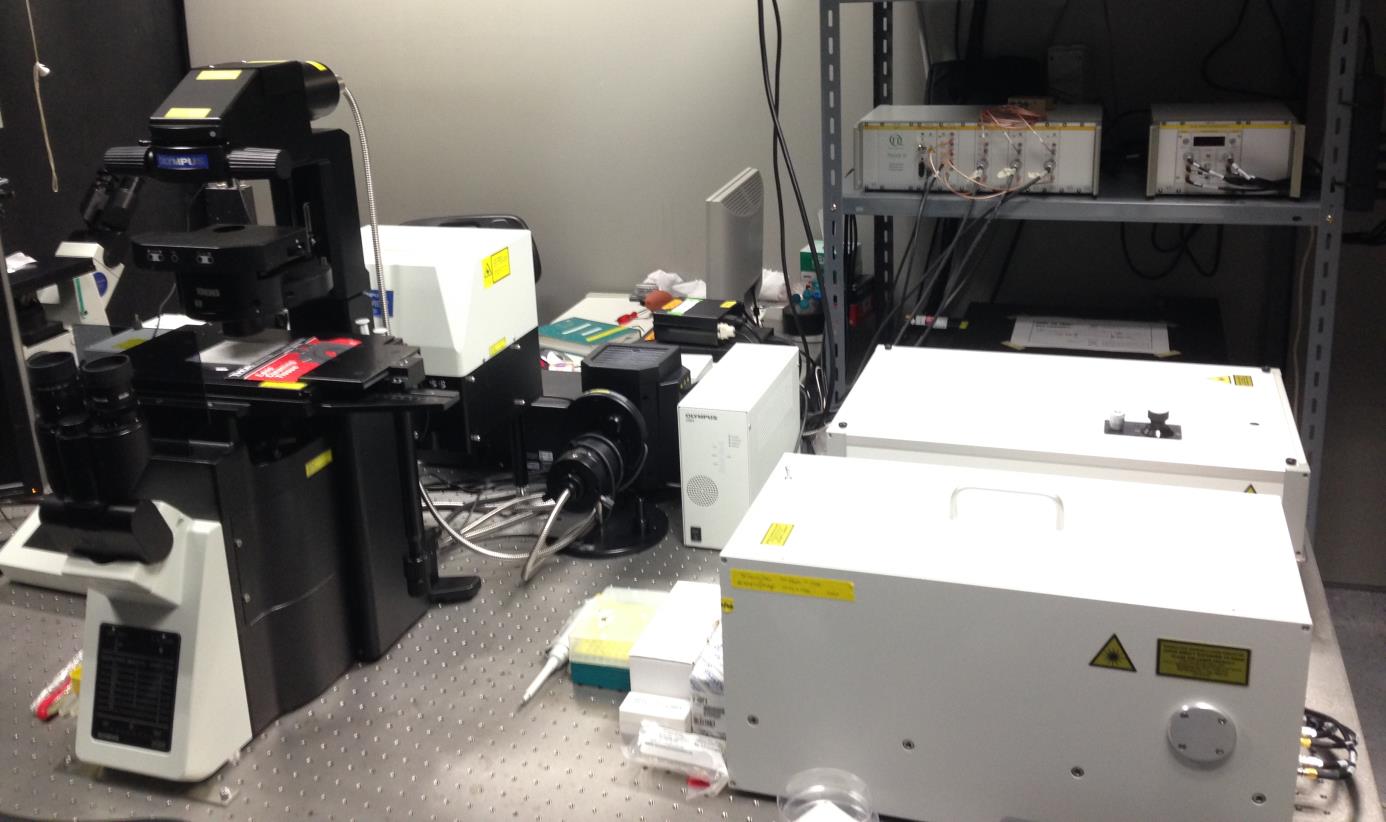
This system in built under ‘PicoQuant LSM upgrade kit’ where an Olympus (IX83) CLSM microscope is combined with PicoQuant lasers, Olympus lasers and PicoQuant detector module.
|
Correlator |
Software |
|
Lasers |
405 nm, 485 nm, 640 nm (pulsed and CW) |
|
Number of Detectors |
2 SPAD (for spectroscopy) and 3 PMT (for imaging) |
|
Measurement type |
Single spot FCS, FCCS, PIE-FCCS, FLCS, sm-FRET, fluorescence lifetime, PCH |
Fluorescence lifetime measurements can be done in this machine which is equipped with pulsed excitation sources (405 nm, 485 nm and 640 nm; pulse width < 100 ps and maximum repetition rate 80 MHz) and a sophisticated time correlated single photon counting (TCSPC) card (TimeHarp 260, PicoQuant). The raw data is saved as the ‘Time Tagged Time Resolved’ (TTTR) format in which each recorded photon is tagged with two characteristic times: i) macrotime, i.e., the time relative to the start of the experiment, and ii) microtime, i.e., time relative to the last excitation pulse. The macrotime and microtime are saved with 50 ns and 25 ps time resolution respectively. The distribution of the microtime of all detected photons (photon arrival time distribution) provides the information of average fluorescence lifetime. Fluorescence lifetime is utilized to measure the time-resolved Förster Resonance Energy Transfer (trFRET) between donor-acceptor pair. In addition, one can measure intensity-based single molecule FRET (smFRET) by comparing the fluorescence intensity at donor and acceptor channels.
More Information on Microscopy Principles
http://micro.magnet.fsu.edu/primer/
(Comprehensive introduction to optical microscopy with many applets and links)
http://www.fcsxpert.com/classroom/
(Site dedicated to FCS with some tutorials)
http://www.olympusconfocal.com/theory/LSCMIntro.pdf
(Article about the basics of confocal microscopy)